Overview
“Hope lies in dreams, in imagination, and in the courage of those who dare to make dreams into reality.” Jonas Salk
Cure Parkinson's (CP) started the international Linked Clinical Trials (iLCT) initiative to repurpose drugs for the treatment of Parkinson's disease (PD). Combining priorities with the Van Andel Institute (VAI), they hold an annual two-day meeting to present, examine, discuss, and prioritize suitable compounds for streamlining into clinical trials specifically for slowing down/stopping/reversing the progression of PD.
I was an invited Parkinson’s patient advocate to the iLCT meeting held at the Van Andel Institute (Grand Rapids, MI, USA) last September 26-27, 2022. Herein is a synopsis of the iLCT program and meeting.
Introduction
“Once you choose hope, anything's possible.” Christopher Reeve
Approximately 90,000 people per year in the USA, 65 or older, will receive a diagnosis of Parkinson’s [1, 2]. A total of up to 10 million people worldwide currently live with the disease. PD symptoms occur gradually over several years, making diagnosing difficult [3, 4]. In PD, the four major symptoms are bradykinesia, rigidity, postural instability, and tremor [5]. In addition to motor symptoms, PD can cause numerous non-motor symptoms [6]. The complex etiology of PD includes genetic mutation, immune system dysfunction, mitochondrial dysfunction, multifactorial environmental factors, neuroinflammation, oxidative stress, and protein aggregation [7-9]. Moreover, this means that halting or slowing PD progression will require a multifaceted therapeutic (or intervention) strategy [10-13]. Furthermore, to this point, PD is not curative.
International Linked Clinical Trials (iLCT) Program for Parkinson’s
“There should be no boundaries to human endeavor. We are all different. However bad life may seem, there is always something you can do, and succeed at. While there’s life, there is hope.” Stephen Hawking, The Theory of Everything
While searching for clues to cure PD, there is also growing hope of devising treatment strategies that promote neuro-restoration/neuroplasticity. To that hopeful means, the premise for the iLCT program for PD is that approved drugs can be studied for additional clinical use through repurposing [14-16]. In other words, can one identify an approach for an already approved compound and retarget that compound for therapeutic intervention in PD? If successful, this would reduce the time and cost of taking this compound through the regulatory evaluation process and, hopefully, approval for treating PD.
This repurposing plan began in 2010 by Cure Parkinson's (CP), and in 2012, the Van Andel Institute (VAI) endorsed the process, which essentially substantiated and validated the iLCT program. The organization of the iLCT is somewhat reminiscent of the umbrella-type grants funded by the National Institutes of Health in the USA that bring together multiple individual projects. Yet, a collective committee governs the oversight. The goal is to bring together noted scientists and clinicians in the field of PD, with whom committee members have agreed to work as a unified team committed to reviewing and prioritizing several lead compounds for repurposing into therapeutic use for PD. The goals and labor plan for the iLCT program are outlined further in Figure 1.

Figure 1. Overview of the iLCT program.
iLCT Dossiers and a Program of Hope
"While I breathe, I hope,” translated from the Latin phrase “Dum spiro spero” is the motto of the U.S. state of South Carolina.
The critical element of the iLCT committee work is fueled by the dossiers prepared for ~20 compounds. First, the compounds described in these dossiers are rigorously reviewed and evaluated. Then after several rounds of discussion, the iLCT committee prioritizes the top substances with information derived from the dossiers. These thoughtful and incredibly well-written dossiers are like mini-grant proposals describing the potential for each compound to be repurposed for PD therapy. These dossiers are not made public, and the final 15 substances are discussed in detail by the committee, ultimately leading to a vote to consider the top 3-5 drugs for PD clinical trials and potential therapies (outlined further in Figure 2). Also present at the meeting and discussions are patient members of the PD research advocate community, who are also given access to dossiers prepared by CP. However, only the iLCT committee can rank and vote on the compounds discussed.
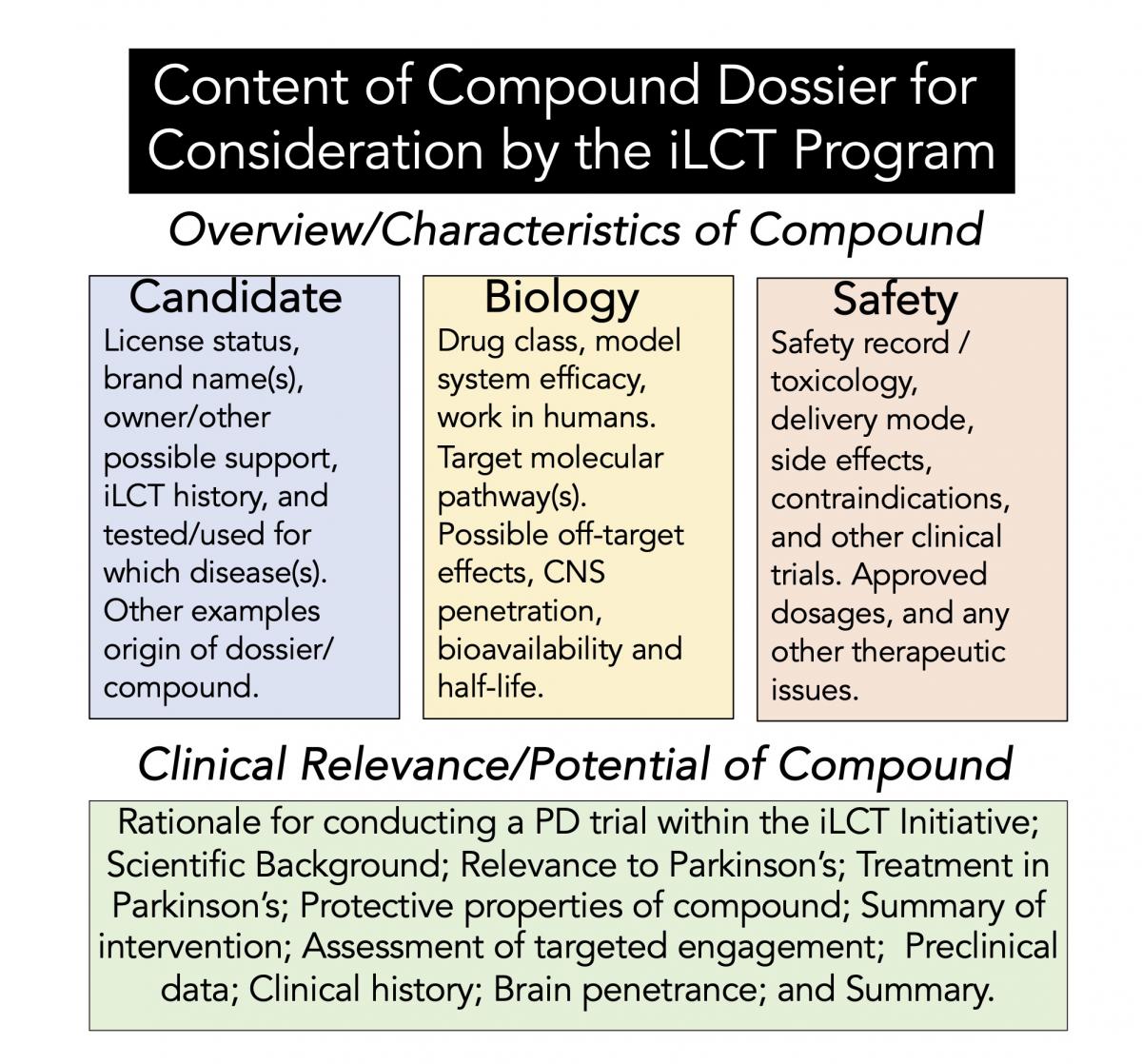
Figure 2. Format of the dossier used in the iLCT program.
The iLCT program has now been in operation for more than 10 years. They have initiated clinical trials for substances with a broad array of molecular targets that might modify or alter the neurological decline found in PD. When a lead compound is prioritized by the iLCT committee, CP and VAI must begin clinical trial studies on that given drug. Thus, in 2023, there have been 20 trials of 17 drugs evaluated by the iLCT committee. Of these, 9 of the agents were prioritized. And there are 20 trials ongoing assessing 17 iLCT evaluated agents in people (person)-with-Parkinson's (PwP), of which 10 drugs have been prioritized.
The first drug prioritized for repurposing by the iLCT was a GLP-1R agonist (Bydureon), which led to a phase II trial that met its primary endpoint [17, 18]. Following this were additional clinical trials with other GLP-1R agonists (Liraglutide, Lixisenatide, and new formulations of Exenatide such as NLY01 and Petron), leading to a Phase III trial with Exenatide in PD [19].
Overall Opinion of the iLCT Meeting
"If we believe that tomorrow will be better, we can bear a hardship today." Thích Nhất Hạnh
I left the meeting with an indelible impression of their work. The iLCT meeting felt like a research conference (linking science and education) combined with a grant review panel (collectively including a final ranked/priority score). The panels' openness and discussions during the presentations were remarkable. Each dossier (the document written describing each individual compound being tested) was given the same importance as the other substances presented; thus, there was no apparent predetermined rank or bias of the dossiers. The room full of experts was made up of equals, giving everyone and anyone the opportunity to discuss or re-visit a point of discussion or contention. And finally, this committee was composed of intelligent, respected, and dedicated scientists and clinicians seeking the most helpful information to rank these substances.
Overall Impression of the iLCT Committee
"The capacity for hope is the most significant fact of life. It provides human beings with a sense of destination and the energy to get started." Norman Cousins
The combined brilliance of the scientists/clinicians assembled in the iLCT committee, and the apparent heart and determination shown by the people behind CP and VAI suggest that PwP should be hopeful for the future treatment of PD.
Conclusions
“Hope is in each of us. For those of us with Parkinson’s, we remain hopeful for new treatments, advances and one day ahead, a cure. But for now, we use courage and determination, mixed with a will to survive, and all held together by a glue we call hope. Stay strong, stay hopeful, stay you.” Frank C. Church
In closing, I was emboldened by the iLCT. Repurposing existing drugs is a reasonable and safer way to identify substances with potential action to treat PD. Clinical trials are both time-consuming and expensive. I encourage the PD community to stay hopeful that one day soon, some beneficial therapeutic compounds for PD will be presented by the iLCT program as spearheaded by CP and VAI.
Acknowledgments: F.C.C. is most appreciative of Dr. Simon Stott, Helen Matthews, and Rick Lay for their insight about CP and the iLCT. F.C.C. thanks Byrd Nichols for useful suggestions in reviewing the blog post/manuscript. F.C.C. acknowledges Dr. Russell R. Broaddus, the Joe W. and Evelyn M. Grisham Distinguished Professor and Department Chair in the Department of Pathology and Laboratory Medicine at UNC School of Medicine, for continued support of this Parkinson’s disease scholarship.
Funding: This work received no external funding.
Ethics statement and declaration: There are no experimental procedures performed in this paper/blog post. There are no human subjects in this work and informed consent is not applicable. The paper submitted here is original work, it has not been published before, and it is not being considered by another journal.
Declaration of competing interest: The author has no conflict of interest to report.
References
[1] Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. Jama 323, 548-560.
[2] Willis AW, Roberts E, Beck JC, Fiske B, Ross W, Savica R, Van Den Eeden SK, Tanner CM, Marras C, Alcalay R, Schwarzschild M, Racette B, Chen H, Church T, Wilson B, Doria JM, on behalf of the Parkinson’s Foundation PG (2022) Incidence of Parkinson disease in North America. npj Parkinson's Disease 8, 170.
[3] Tolosa E, Garrido A, Scholz SW, Poewe W (2021) Challenges in the diagnosis of Parkinson's disease. The Lancet Neurology 20, 385-397.
[4] Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. Journal of neurology, neurosurgery & psychiatry 79, 368-376.
[5] Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RM, Seppi K, Coelho M, Sampaio C, Committee MDSEBM (2018) International Parkinson and movement disorder society evidence‐based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Movement Disorders 33, 1248-1266.
[6] Pfeiffer RF (2016) Non-motor symptoms in Parkinson's disease. Parkinsonism & related disorders 22, S119-S122.
[7] Olanow C, Tatton W (1999) Etiology and pathogenesis of Parkinson's disease. Annual review of neuroscience 22, 123.
[8] Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson's disease. Movement disorders 26, 1049-1055.
[9] Wirdefeldt K, Adami H-O, Cole P, Trichopoulos D, Mandel J (2011) Epidemiology and etiology of Parkinson’s disease: a review of the evidence. European journal of epidemiology 26, 1-58.
[10] Church FC (2021) Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 11, 612.
[11] Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. Jama 311, 1670-1683.
[12] Hribar CA, Cobbold PH, Church FC (2020) Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain sciences 10, 284.
[13] Hall M-FE, Church FC (2020) Exercise for older adults improves the quality of life in Parkinson’s disease and potentially enhances the immune response to COVID-19. Brain Sciences 10, 612.
[14] Stott SR, Wyse RK, Brundin P (2021) Drug repurposing for Parkinson’s disease: the international linked clinical trials experience. Frontiers in Neuroscience 15, 653377.
[15] Brundin P, Wyse RK (2019) The linked clinical trials initiative (LCT) for Parkinson's disease. European Journal of Neuroscience 49, 307-315.
[16] Latif K, Ullah A, Shkodina AD, Boiko DI, Rafique Z, Alghamdi BS, Alfaleh MA, Ashraf GM (2022) Drug reprofiling history and potential therapies against Parkinson’s disease. Frontiers in Pharmacology 13, 1028356.
[17] Foltynie T, Athauda D (2020) Repurposing anti-diabetic drugs for the treatment of Parkinson's disease: rationale and clinical experience. Progress in Brain Research 252, 493-523.
[18] Foltynie T, Aviles-Olmos I (2014) Exenatide as a potential treatment for patients with Parkinson's disease: first steps into the clinic. Alzheimer's & Dementia 10, S38-S46.
[19] Vijiaratnam N, Girges C, Auld G, Chau M, Maclagan K, King A, Skene S, Chowdhury K, Hibbert S, Morris H (2021) Exenatide once weekly over 2 years as a potential disease-modifying treatment for Parkinson’s disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: the ‘Exenatide-PD3’study. BMJ open 11, e047993.









- Comment
|