Abstract. Multiple system atrophy (MSA) is a rare, rapidly progressive neurodegenerative disorder of the adulthood, characterized by autonomic failure, parkinsonian and cerebellar features in various combinations. Distinguishing MSA from common clinical look-alikes such as Parkinson´s disease, other atypical parkinsonian disorders or alternative causes of sporadic adult-onset cerebellar ataxia may be difficult, especially at early disease stages. Nonetheless, some simple and cost-effective screening tools help detecting important red flags guiding towards a MSA diagnosis. Here we outline which clinical pearls and bedside tests may disclose autonomic dysfunction in multiple domains, enabling an early MSA diagnosis and, even more importantly, personalized treatment.
THE CLINICAL DILEMMA
Multiple system atrophy (MSA) is a rare, rapidly progressive neurodegenerative disorder of the adult-hood, presenting with autonomic failure, poorly L-dopa responsive parkinsonism and cerebellar ataxia in various combinations [1]. Screening for autonomic dysfunction is important both for a timely MSA diagnosis and personalizing treatment strategies [2], but the availability of diagnostic autonomic function laboratories is often limited. Here we review some bedside clinical pearls and screening tests that help disclosing autonomic failure in patients with suspected MSA.
BEDSIDE ASSESSMENT OF AUTONOMIC DYSFUNCTION
History taking
In MSA, autonomic dysfunction is typically severe, begins early in the disease course and affects multiple domains.
Neurogenic orthostatic hypotension and urogenital dysfunction represent core MSA diagnostic features [1, 3]. Symptoms suggestive of orthostatic intolerance such as syncope, light-headedness, blurred/darkened vision, troubles concentrating, neck, shoulder (so called “coat-hanger”) and low-back pain, typically develop upon standing and improve after sitting or lying down. Such symptoms are often reported after large meals, pointing towards post-prandial hypotension, and may be exacerbated by levodopa intake.
Lower urinary tract symptoms (LUTS) are common features already at early MSA stages. History taking should therefore address both storage (urinary urgency, frequency, urge incontinence, and nocturia) and voiding disturbances (hesitancy, incomplete bladder emptying, poor and intermittent urine stream). Unexplained erectile dysfunction in men younger than 60 is another suggestive MSA red flag. Stridor is a high-pitched inspiratory sound, which is highly distinctive of MSA. Mimicking stridor with the physician’s voice or using audio recordings may enable caregivers recognizing it (an audio recording of stridor can be found under [4]).
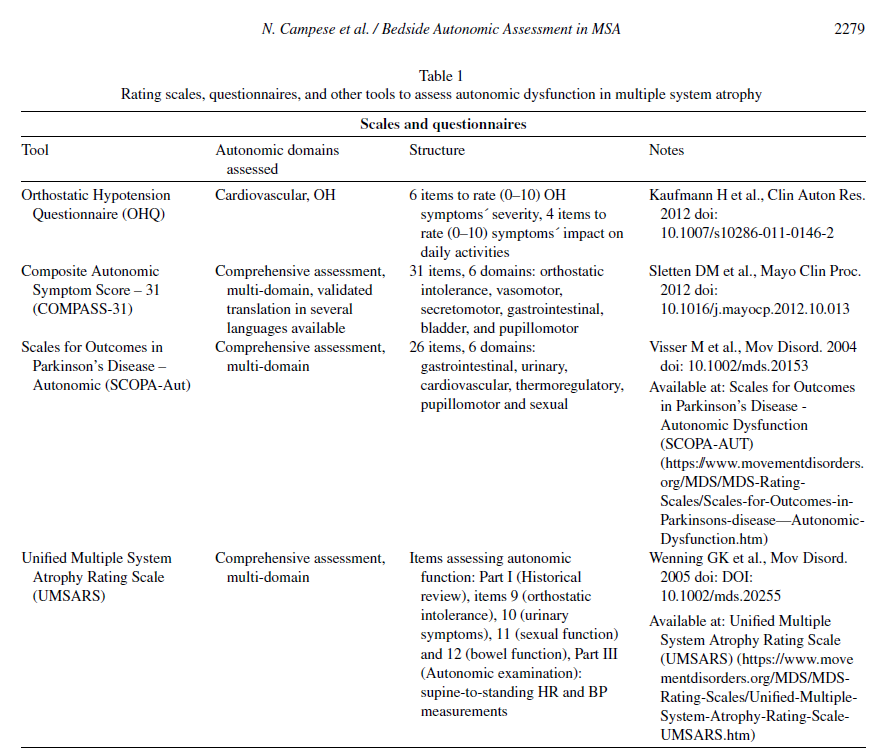
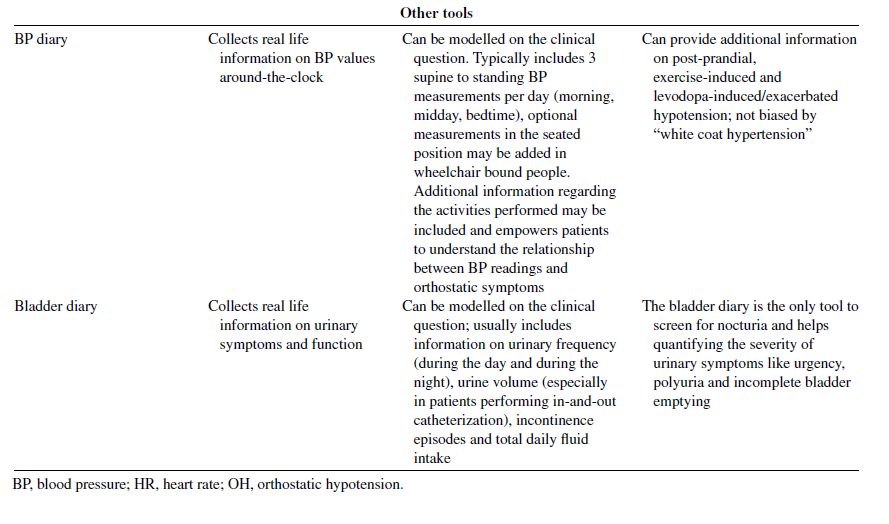
Patient-reported outcomes tools and rating scales (Table 1) can assist physicians in the systematic assessment of autonomic complaints and in quantifying their burden and impact on quality of life. Self-completed blood pressure and bladder diaries may provide additional real-life information. Their use is particularly recommended in those low-resources contexts, where access to second-level examinations (e.g., tilt-table and other cardiovascular autonomic function tests, 24-hour ambulatory blood pressure measurements and urodynamic studies) is limited.
Physical examination
In people with suspected MSA, the neurological examination is often biased towards its core motor features. Nonetheless, a careful physical examination may disclose cold, discolored hands and feet (Fig. 1A–C) showing delayed capillary refill after digital pressure (Fig. 1D). The so-called “cold hands and cold feet sign” indicates insufficient sympathetic control over cutaneous blood vessels and represents an important MSA red flag [3, 5]. Skin examination may disclose further indirect signs of impaired sweating, such as dry and chapped skin. Both stridor and inspiratory sighs may be heard during the examination.
Bedside assessments
The standing test is a simple and cost-effective tool to screen for orthostatic hypotension (OH) at bedside. It is performed with an arm-cuff sphygmomanometer by measuring supine-to-standing heart rate (HR) and blood pressure (BP) changes 1, 3, and 5 (or 10) minutes upon standing. The test indicates classic OH in case of a sustained BP fall ≥20 mmHg systolic and/or ≥10 mmHg diastolic within 3 minutes upon standing. Prolonging the orthostatic challenge beyond 3 minutes may disclose delayed OH. Delayed OH is considered a beginning form of OH, which may progress to classic OH over time [3]. Since OH may often remain asymptomatic (a, to date not fully understood phenomenon called “hypotensive unawareness” [6, 7]), actively screening for OH is particularly important in patients with recurrent unexplained falls. The calculation of the neurogenic-OH (nOH) ratio, i.e., the ratio between the increase in heart rate and the fall in systolic BP upon standing, may help differentiating neurogenic from non-neurogenic causes of OH [8].
This ratio cannot be applied to patients, whose heart rate is influenced by other factors like cardiac arrhythmia, pacemakers or -blocker therapy [9]. The diagnostic accuracy of both the standing test and the nOH ratio is however suboptimal, especially in milder forms of cardiovascular autonomic failure. These bedside tests should be therefore intended as screening measures to identify those patients, who may benefit from referral to specialized centers for tilt test and other cardiovascular autonomic function tests. A negative standing test does not also completely rule out nOH, so that patients with a strong clinical suspicion should be equally referred for an in-depth autonomic assessment.
In subject reporting urinary complaints, urinary tract infections should be ruled out first by means of urine dipsticks. Post-void bladder ultrasound or in-and-out catheterization may point out voiding disturbances in case of a residual post-void urine volume 100 ml and identify patients with an indication for urodynamic studies [3].
DISCUSSION AND CONCLUSIONS
Differentiating MSA from its clinical lookalikes can be difficult, especially at early disease stages. Cardiovascular and urogenital autonomic failure are frequent both in MSA and Parkinson’s disease, but their development early in the disease course rather points towards a MSA diagnosis [2, 10]. Tauopathies, such as progressive supranuclear palsy and corticobasal degeneration, may mimic some core motor features of MSA, but the presence of severe, multi-domain autonomic failure is an exclusion diagnostic criterion for both disorders [11, 12]. In sporadic adult-onset ataxias other than MSA, only mild forms of autonomic failure occur, in any [2].
Detecting early and severe autonomic dysfunction in patients presenting with rapidly progressive parkinsonism or cerebellar ataxia is important for an accurate diagnosis and management of MSA. Simple and cost-effective tools may be useful to disclose autonomic dysfunction at bedside, timely identifying those patients who may benefit from an in-depth differential diagnostic work-up.

Patient consent
We declare that written informed consent for both video/picture recording and their publication was obtained from the patient and can be provided upon request; all information has been managed confidentially and in accordance to current European data protection and privacy regulations. No patient-related information enabling subject´s identification is disclosed. The Innsbruck Ethical Committee approved the case report (EK Nr: 1233/2021).
ACKNOWLEDGMENTS
We would like to thank our patient for agreeing to the pictures/video collection and their publication in the present work.
We express our gratitude to Dr. Jean Pierre Ndayisaba, PhD (Department of Neurology, Medical University of Innsbruck, Innsbruck – Austria) for the technical support in editing the multimedia material.
Academic study without external financial support. N.C. is supported by a mobility grant of the European Academy of Neurology (EAN), F.L. by the US MSA Coalition and Dr Johannes Tuba Stiftung.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/ 10.3233/JPD-223357.
REFERENCES
[1] Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372, 249-263.
[2] Leys F, Wenning GK, Fanciulli A (2022) The role of cardiovascular autonomic failure in the differential diagnosis of alpha-synucleinopathies. Neurol Sci 43, 187-198.
[3] Wenning GK, Stankovic I, Vignatelli L, Fanciulli A, Calandra-Buonaura G, Seppi K, Palma JA, Meissner WG, Krismer F, Berg D, Cortelli P, Freeman R, Halliday G, H¨oglinger G, Lang A, Ling H, Litvan I, Low P, Miki Y, Panicker J, Pellecchia MT, Quinn N, Sakakibara R, Stamelou M, Tolosa E, Tsuji S, Warner T, Poewe W, Kaufmann H (2022) The Movement Disorder Society criteria for the diagnosis of multiple system atrophy. Mov Disord 37, 1131-1148.
[4] Cortelli P, Calandra-Buonaura G, Benarroch EE, Giannini G, Iranzo A, Low PA, Martinelli P, Provini F, Quinn N, Tolosa E, Wenning GK, Abbruzzese G, Bower P, Alfonsi E, Ghorayeb I, Ozawa T, Pacchetti C, Pozzi NG, Vicini C, Antonini A, Bhatia KP, Bonavita J, Kaufmann H, Pellecchia MT, Pizzorni N, Schindler A, Tison F, Vignatelli L, Meissner WG (2019) Stridor in multiple system atrophy: Consensus statement on diagnosis, prognosis, and treatment. Neurology 93, 630-639.
[5] Klein C, Brown R, Wenning G, Quinn N (1997) The ”cold hands sign” in multiple system atrophy. Mov Disord 12, 514-518.
[6] van Dijk JG, van Rossum IA, Thijs RD (2020) Timing of circulatory and neurological events in syncope. Front Cardiovasc Med 7, 36.
[7] Tipton PW, Cheshire WP (2020) Mechanisms underlying unawareness of neurogenic orthostatic hypotension. Clin Auton Res 30, 279-281.
[8] Fanciulli A, Kerer K, Leys F, Seppi K, Kaufmann H, Norcliffe-Kaufmann L, Wenning G (2020) Validation of the neurogenic orthostatic hypotension ratio with active stand-ing. Ann Neurol 88, 643-645.
[9] Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, Peltier AC, Singer W, Low PA, Goldstein DS, Gibbons CH, Freeman R, Robertson D, Autonomic Disorders Consortium (2018) Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol 83, 522-531.
[10] Fanciulli A, Goebel G, Lazzeri G, Scherfler C, Gizewski ER, Granata R, Kiss G, Strano S, Colosimo C, Pontieri FE, Kaufmann H, Seppi K, Poewe W, Wenning GK (2019) Early distinction of Parkinson-variant multiple system atrophy from Parkinson’s disease. Mov Disord 34, 440-441.
[11] Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, Movement Disorder Society-endorsed PSP Study Group (2017) Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 32, 853-864.
[12] Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer ALD, DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Tr¨oster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496-503.
-----------
Read the full article published in the Journal of Parkinson’s Disease here:
Bedside Assessment of Autonomic Dysfunction in Multiple System Atrophy, by Nicole Campese, Fabian Leys, Gregor K.Wenning, Alessandra Fanciulli, J Parkinsons Dis, vol. 12, no. 7, pp. 2277-2281, 2022, DOI: 10.3233/JPD-223357










- Comment
|